New way to examine virus proteins for antimicrobial properties may aid in combating antibiotic resistance
April 01, 2023 | Microbiology Lab
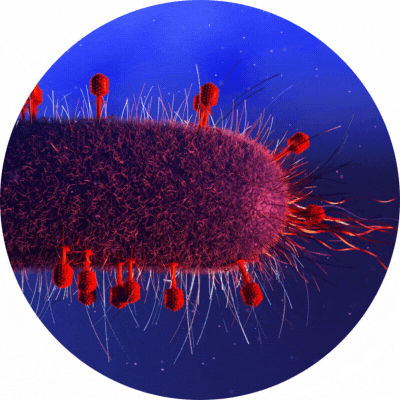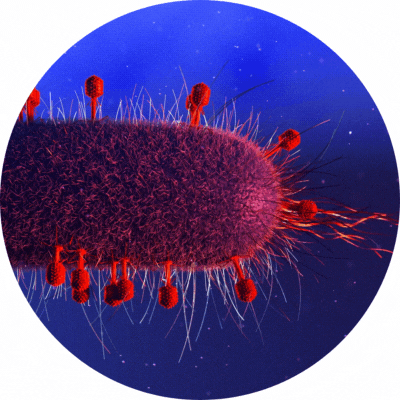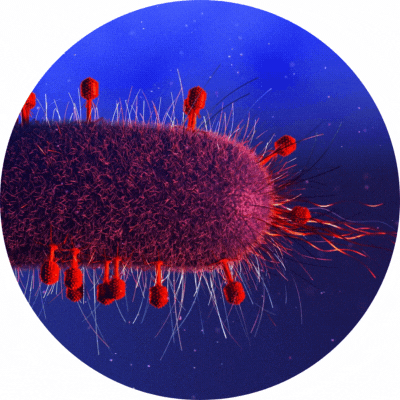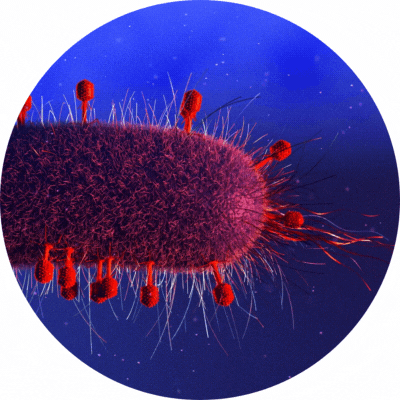
Antimicrobial resistance continues to be a major concern in the medical field the status quo. Various research endeavors have been centered on investigating bacteria-killing techniques through bacteriophages (phages), viruses that are found to infect bacteria.
A novel genetic screening approach invented by Lawrence Berkeley National Laboratory is in the works to identify the part of the bacterial cell targeted by a potent bacteriophage weapon called “single-gene lysis protein” (Sgls).
“With rising antibiotic resistance, we urgently need antibiotic alternatives. Some of the smallest phages that we know of code for single-gene lysis proteins (Sgls), also known as ‘protein antibiotics,’ to inhibit key components of bacterial cell wall production that, when disrupted, consistently kill the cell.”, says Vivek Mutalik, a staff scientist in the Berkely Lab’s Biosciences Area and co-senior author the novel approach.
The current undertaking is barred by the problem of isolating a single page from the environment and assessing which microbe it targets and how. Mutalik’s team bridges the gap on this by inventing Dual-Barcoded Shotgun Expression Library Sequencing (Dub-seq). The technology allows scientists to investigate unknown genes function by employing a coded library of DNA fragments without the need for culturation. Through the process, they are able to conduct a detailed characterization of the function of one of the six Sgls from six phages that infect different bacteria.
Results of the study reveal that the Sgl proteins target pathways for cell wall building in bacteria. And since Sgl proteins attack such areas, they become capable of killing bacteria other than the phage’s target strain–tantamount to their potential use in antibiotics.
“Phages are extraordinary innovators when it comes to destroying bacteria. We’re really excited to uncover novel bacterial pathogen-targeting mechanisms that could be leveraged into therapies,” says Benjamin Adler, first author and postdoctoral fellow in Jennifer Doudna’s lab at the University of California Berkeley.
With the new approach, the team is keen to apply such in thousands of single-gene lysis-producing phases so they may be characterized in the environmental samples collected from the ocean, soils, and even the human gut. Their ultimate goal is to make it an inspiration for the next breakthrough medicine in the near future.